
Walt pouring Dietyl ether in a cook session.
Diethyl ether, also known as ethyl ether, sulfuric ether, simply ether, or ethoxyethane, is an organic compound in the ether class. Diethyl ether is a common laboratory solvent often used for liquid-liquid extractions (generally called solvent extractions). It has limited solubility in water (6.05 g/100 mL at 25 °C.) It is a colorless, highly volatile flammable liquid. It is commonly used as a solvent and was once used as a general anesthetic. It has narcotic properties and has been known to cause temporary psychological addiction, sometimes referred to as etheromania. Diethyl ether forms an organic layer above an aqueous layer, so Walt could have been using it as a potential solvent for the reaction if phenylacetic acid turned out to be less soluble.
Production
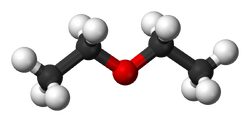
Ball and stick model of diethyl ether.
Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Ethanol is mixed with a strong acid, typically sulfuric acid, H2SO4. The acid dissociates in the aqueous environment producing hydronium ions, H3O+. A hydrogen ion protonates the electronegative oxygen atom of the ethanol, giving the ethanol molecule a positive charge:
- CH3CH2OH + H3O+ → CH3CH2OH2+ + H2O
A nucleophilic oxygen atom of unprotonated ethanol displaces a water molecule from the protonated (electrophilic) ethanol molecule, producing water, a hydrogen ion and diethyl ether.
- CH3CH2OH2+ + CH3CH2OH → H2O + H+ + CH3CH2OCH2CH3
This reaction must be carried out at temperatures lower than 150 °C in order to ensure that an elimination product (ethylene) is not a product of the reaction. At higher temperatures, ethanol will dehydrate to form ethylene. The reaction to make diethyl ether is reversible, so eventually an Chemical equilibrium between reactants and products is achieved. Another reaction that can be used for the preparation of ethers is the Williamson ether synthesis, in which an alkoxide (produced by dissolving an alkali metal in the alcohol to be used) performs a nucleophilic substitution upon an alkyl halide.
